
2015 Edition Cures Act Update
Posted on March 22, 2020
ONC (the Office of the National Coordinator for Healthcare IT) issued its long-anticipated new Final Rule for the ONC HIT Certification Program on March 9, 2020 titled “21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program”. The ONC HIT Certification Program regulates how software products such as EHR, eRx, Direct messaging, patient portal etc. are certified for use in CMS programs such as MIPS. This article is intended to assist practices interpret what this change in certification rules means to them.
The article attempts to strike a balance between readability and accuracy on an inherently complex regulatory topic. To that end, highlights of particular interest to practices are marked “P1”, “P2” etc. and the reader may quickly review just these points.
The reader is encouraged to review a previous blog article “The CEHRT / Base EHR Gap” to understand the somewhat confusing relationship between the regulatory terms “CEHRT” as defined by CMS versus “2015 Edition Base EHR” as defined by ONC.
Retirement of “2014 Edition”
The “2014 Edition” had originally been issued by ONC in a 2012 Final Rule on the same day as the matching CMS Final Rule for Meaningful Use Stage 2. In those early days of the ONC HIT Certification Program, ONC’s primary focus had been to support its sister agency; the Edition name had been based on the year that CMS had intended to make use of that Edition mandatory.
As it later turned out, CMS waffled on making 2014 Edition mandatory in 2014! More than midway through that year, with what CMS considered an unacceptably low real-world adoption rate for 2014 Edition CEHRT, CMS offered a reprieve and allowed practices to attest to Stage 1 for that last year if they were still using 2011 Edition and their HIT vendor was not able to implement 2014 Edition in a timely manner. Practices who had expedited the major upgrade to 2014 Edition and were now forced to attest to Stage 2 might have considered this action unfair.
This CMS action also apparently frustrated ONC. ONC had (and continues to have) an ambitious program to advance Healthcare IT nationwide via forcing new and evolving technical standards such as Direct messaging on vendors and practices alike, and CMS was delaying their agenda. The increasing tension between CMS and ONC became visible the following year when ONC announced a new naming convention for its certification editions: these would no longer be named after the year that CMS first planned to make the edition mandatory but rather after the year in which ONC issued the edition. Thus the name for “2015 Edition” – it was issued in 2015. The astute reader may notice that, had this naming convention been in effect earlier, the “2014 Edition” would have been named the “2012 Edition” (and the “2011 Edition” the “2009 Edition”). As it later turned out, CMS continued to change its mind regarding the year the 2015 Edition would become mandatory, allowing it to drift from 2017 to 2018 and eventually to 2019.
As late as 2018, 2014 Edition CEHRT was acceptable for CMS Programs such as MIPS, but now ONC removed the very definition of 2014 Edition from the Federal Register and immediately expunged all references to it from its Certified Healthcare IT Product List (https://chpl.healthit.gov):
- P1: ONC removed all 2014 Edition certified products from its CHPL website as of March 9, 2020 .
It should now be entirely clear to any practice still using what had been 2014 Edition certified software that this software is no longer holds any certification status.
The “2015 Edition Cures Update”
Having established a new naming convention for its certification Editions back in 2015, ONC has now abandoned that naming convention at this first opportunity. Despite the major changes in the certification criteria being introduced (see below), ONC did not name this the “2020 Edition”, instead redefining the “2015 Edition” despite the inevitable confusion!
Why? A clear advantage for ONC is that this allows ONC, rather than CMS, to determine when use of the edition becomes mandatory. After all, CMS’s definition of “CEHRT” currently relies on ONC’s definition of “2015 Edition Base EHR”; by simply redefining the term “2015 Edition Base EHR” and imposing “Conditions and Maintenance of Certification Requirements” on HIT vendors, ONC has put itself in the driver’s seat and no longer subject at the mercy of CMS rule-making; ONC’s agenda for new technology standards is back on the fast track!
Nevertheless, even ONC has a need to distinguish the “new” 2015 Edition from the “old”:
- P2: ONC will label products certified to the updated 2015 Edition as “2015 Edition Cures Update” on its CHPL website.
This “2015 Edition Cures Update” retires some certification criteria, modifies others, and adds 2 important new criteria. The reader who wants to know all the details is referred to Table 1 of the ONC Final Rule which summarizes all of these changes, but the purpose of this article is to highlight key changes which have a particular impact on practices. These changes are not all mandated immediately, rather ONC specifies two key future dates:
- P3: by March, 2022, HIT vendors are required to make technology certified to “2015 Edition Cures Update” criteria required for “2015 Edition Base EHR” available to their customers, including:
- P3-A: USCDI – use of the “United States Core Data for Interoperability” standard to replace the 2015 Edition “Common Clinical Data Set” (CCDS) – see Table 1 below for a list of affected certification criteria and the explanation of what additional data has been added;
- P3-B: NCPDP – use of the advanced National Council for Prescription Drug Programs standard for Electronic Prescribing, as related to certification criterion § 170.315(b)(3) and affecting the PI Measure for e-Prescribing; and
- P3-C: FHIR API – the “Standardized API for Patient and Population Services” – a new certification criterion § 170.315 (g)(10) requiring the HL7 FHIR technical standard for single-patient and multi-patient access to health information.
- P4: by March, 2023, as a condition of maintenance of certification, products which electronically store EHI (Electronic Health Information) are required to certify to a new criterion “EHI Ex
The timelines of the recent ONC certification editions are summarized in the following diagram, based on the assumption that ONC will stick to its schedule:
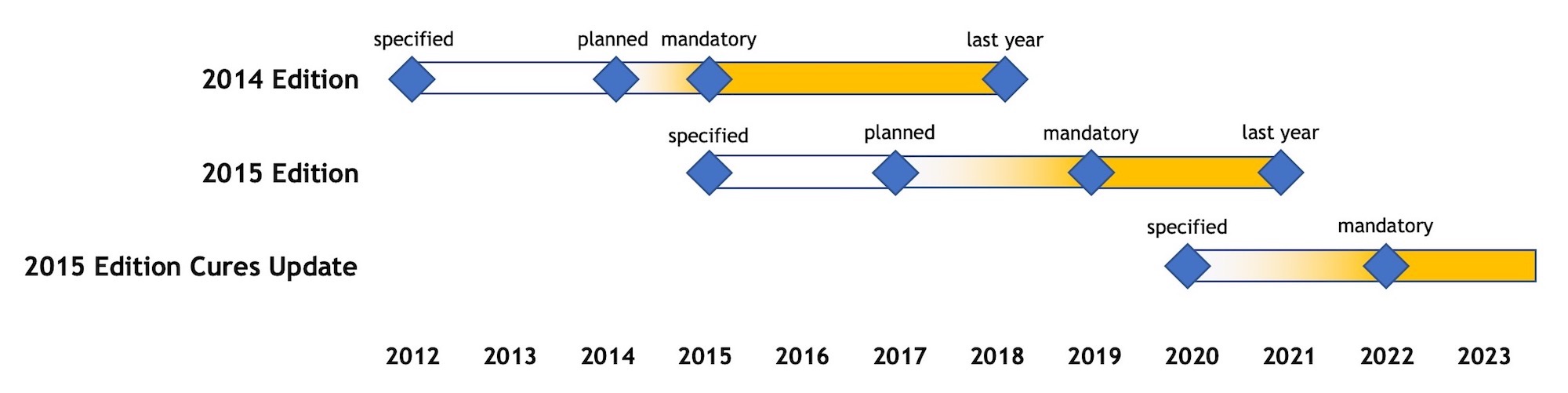
Diagram 1 – Timeline of ONC Certification Editions
We will go through these key changes one after another.
UCSDI Related Certified Capabilities
The USCDI (“United States Core Data for Interoperability”) standard extends the 2015 Edition Common Clinical Data Set with additional mandatory data elements and will affect the following certified capabilities as related to MIPS and other CMS programs:
| Certification Criteria | Reference | Impact on CMS Programs |
| Transitions of Care | § 170.315(b)(1) | PI Measures: (a) Support Electronic Referral Loops by Sending Health Information, and (b) Support Electronic Referral Loops by Receiving and Incorporating Health Information |
| Clinical information reconciliation and incorporation | § 170.315(b)(2) | PI Measure: Support Electronic Referral Loops by Receiving and Incorporating Health Information |
| View, Download, and Transmit to 3rd Party | § 170.315(e)(1) | PI Measure: Provide Patients Electronic Access to Their Health Information |
| Transmission to public health agencies — electronic case reporting | § 170.315(f)(5) | PI Measure: Electronic Case Reporting |
| Consolidated CDA creation performance | § 170.315(g)(6) | Mandatory for CEHRT |
| Application Access – All Data Request | § 170.315(g)(9) | PI Measure:
Provide Patients Electronic Access to Their Health Information |
Table 1 – Certification Criteria Affected by UCSDI Standard
UCSDI will continue to be based on the HL7 C-CDA (Consolidated Clinical Document Architecture) standard.
- P5: 2015 Edition Cures Update CEHRT will be required to provide a richer set of mandatory data for exchange of data with outside providers, patients and certain registries; this will become mandatory in 2022.
The USCDI data extensions relative to the 2015 Edition Common Clinical Data Set are as follows:
- Demographics
- Current / Previous Address
- Phone Number / Type
- E-mail Address
- Pediatric Vital Signs
- Clinical Notes / Free Text ✓ Discharge Summary Note
- History & Physical
- Progress Note
- Consultation Note
- Imaging Narrative
- Laboratory Report Narrative
- Pathology Report Narrative
- Procedures Note
- Provenance
- Author’s Time Stamp
- Author’s Organization
- “Medication Allergies” renamed as “Allergies and Intolerances” to broaden scope to non-medication substances
The 8 note types were ones identified by the Argonaut Project and are also included in the HL7 C-CDA standard. ONC provides clear guidance that these “notes” are in plain text format for maximum interoperability and should not be interpreted or associated with C-CDA document templates that may share the same name.
Practices should look for their HIT vendor to support easily incorporating the various types of free text notes received from outside providers of care into their patient charts, with provenance information included so as to provide proper clinical context. This ability will hopefully be available with the 2015 Edition Cures Update version of their CEHRT.
While these are welcome changes, it is unfortunate that ONC has not yet found a way to require specialty-specific data – for example visual acuities, intra-ocular pressures and spectacle prescriptions for ophthalmology and optometry; such information would be of critical interest for patients as well as care coordination.
FHIR API
The new FHIR API technology required by the new certification criterion § 170.315 (g)(10) should have a major impact on the way patients access their health information. It is reasonable to assume that the new ONC rule will drive a transition from patient portal technology to an ecosystem of widely available patient mobile apps and a much greater percentage of patients accessing their health information electronically. Although 2015 Edition certification and the PI Measure “Provide Patients Electronic Access to Their Health Information” already require a “Patient Access API”, this has not resulted in significant real-world impact because HIT vendors are currently allowed to maintain an entirely proprietary API. With different CEHRT products having different APIs, third-party patient mobile apps are not yet a reality, but standardization to a hopefully plug-and-play level should ensure that such mobile apps will be widely available in the future and work across all 2015 Edition Cures Update CEHRT.
- P6: 2015 Edition Cures Update CEHRT will be required to include a standardized patient access API, so that patients may use their smartphones instead of a patient portal to access their health information; this new technology will become mandatory in 2022.
Since MIPS requires practices to attest to “Prevention of Information Blocking”, it is recommended that, once a FHIR API is available, the practice plan to test their Patient Access API using at least one such third-party mobile app and follow up with their CEHRT vendor in case of issues.
Besides the FHIR API providing a service to allow a patient to access his/her health information, it also will provide a service to retrieve health information for multiple patients, which may be of interest, for example, for future electronic integration of registries with a practice’s CEHRT.
EHI Export
To meet the Cures Act’s goal of “complete access … and use of all electronically accessible health information”, ONC is replacing the current “Data Export” certification criterion § 170.315(b)(6) with a new more comprehensive “EHI Export” intended to be complementary to the new FHIR API and which will become mandatory within 36 months of the Final Rule’s publication date. EHI Export will be required of any certified product which electronic stores EHI (Electronic Health Information) and such export must include all EHI the product is able to store at the time of its certification..
Although the old “Data Export” (which was defined in terms of the Common Clinical Data Set and required export of C-CDA files) was part of the definition of “2015 Edition Base EHR”, the 2015 Edition Cures Update does not include “EHI Export” as part of the definition of “2015 Edition Base EHR”. Instead, as already stated, EHI Export will be required of any certified product which electronic stores EHI.
EHI Export must be available for a single patient or a patient population. The HIT vendor is free to define a suitable proprietary export format; ONC does not reference any technical standard for this purpose; however, the HIT vendor is required to publish the technical specifications of its export format so that it may be used by third-parties.
The EHI Export capability of any product must export all EHI data items electronically stored by that product, relative to the version of the product at the time of certification. This is a much broader requirement than the old “Data Export” which merely required exporting data items in the Common Clinical Data Set.
- P7: 2015 Edition Cures Update CEHRT will be required to include comprehensive export capability, so that practices are guaranteed to have full access to their data; this new technology will become mandatory in 2023.
The above is simply a restatement of P4. A clear benefit to the practice of EHI Export will be to assist migrating its EHI from one CEHRT platform to another.
Summary and Conclusion
ONC will continue to force new and evolving technical standards such as FHIR on vendors and practices alike. These new IT capabilities should provide benefits to practices and their patients in the future, but also represent new challenges. Small practices are often not prepared to deal with these rapid changes in information technology and certification rules as effectively as enterprise health care delivery organizations, due to an economy of scale issue, and blind reliance on the CEHRT vendors is like asking the fox to guard the henhouse. Consequently, small practices may want to consider assistance from a professional consulting group such as HiQ Services to guide them through their selection and effective use of CEHRT.
Recent Posts
- 2021 MIPS Final Rule – Practical Information and Strategic Issues December 7, 2020
- HiQ Adds Platinum Tier to MIPS EssentialsTM Service September 21, 2020
- 2021 MIPS Proposed Rule August 7, 2020
- Waiting for MVPs June 25, 2020
- Webinar – Interoperability and the Practice – June 24, July 8, & Aug 5,2020 May 11, 2020
